In 2016, medical gadget large Abbott issued a recall for its MitraClip cardiac gadget — “a Class I recall, the most serious type,” the FDA mentioned.
“Use of this device may cause serious injuries or death,” an FDA discover in regards to the recall mentioned.
However neither the producer nor the FDA really recalled the gadget or suspended its use. They allowed docs to proceed implanting the clips in leaky coronary heart valves in what has turn out to be a typical process.
In a discover, the producer defined, “Abbott is not removing product from commercial distribution.” Reasonably, Abbott revised directions to be used and required docs who implant the clips to endure coaching.
On the subject of medical gadgets, recollects can embody not solely “removals,” during which the gadget is faraway from the place it’s used or bought, but additionally “corrections,” which tackle the issue within the subject — as an illustration, by repairing, adjusting, relabeling, or inspecting a tool.
“It’s very oxymoronic,” mentioned Rita Redberg, a heart specialist on the College of California-San Francisco and former editor-in-chief of the journal JAMA Inside Medication. “A recall makes it sound like it’s recalled. But that is not actually what it means.”
Although the FDA and federal rules name these actions recollects, they is perhaps described extra aptly as “non-recalls.” They usually have occurred repeatedly in recent times. As an illustration, along with different Abbott gadgets, merchandise made by Medtronic, Abiomed, and Getinge have had recollects that left them in use.
Safeguarding the Public
Remembers that depart what the FDA identifies as doubtlessly harmful merchandise within the market can increase the query: Do they do sufficient to guard the general public?
There are different methods to deal with recollects. In bulletins about merchandise as various as crib bumpers, pool drain covers, bicycle helmets, and low mugs, the Shopper Product Security Fee routinely alerts customers to cease utilizing recalled merchandise and get in touch with the producers for refunds, repairs, or replacements. The Nationwide Freeway Site visitors Security Administration recurrently advises customers to deliver recalled automobiles again to the vendor to have them mounted. When the U.S. Division of Agriculture and the FDA announce meals recollects, they routinely inform customers to return or discard the meals.
In some instances, a medical gadget that’s the topic of a recall might be stored in the marketplace safely as a result of there’s a easy repair, mentioned Sanket Dhruva, a heart specialist and an affiliate professor at UCSF who has studied FDA oversight of gadgets. In different instances, recollects that don’t take away gadgets from the market can present unwarranted reassurance and depart the general public in danger, Dhruva mentioned.
From 2019 by 2023, there have been 338 Class I medical gadget recollects, 164 of which had been corrections and 174 of which had been removals, FDA spokesperson Amanda Hils mentioned.
Some merchandise endure recall after recall whereas they continue to be in the marketplace. Merchandise within the MitraClip line have been the topic of three rounds of recollects, none of which eliminated gadgets from use.
“When deciding whether a recall warrants device removal from the field, the FDA considers the frequency and severity of adverse events, effectiveness of the corrective actions that have been executed, and the benefits and risks of preserving patient access to the device,” FDA spokesperson Audra Harrison mentioned.
The place recalled gadgets have already been implanted, “removal” doesn’t essentially imply eradicating them from sufferers’ our bodies. “When an implanted device has the potential to fail unexpectedly, companies often tell doctors to contact their patients to discuss the risk of removing the device compared to the risk of leaving it in place,” the FDA web site says.
The FDA allowed the recalled MitraClip gadgets to stay in use “because the agency believed that the overall benefits of the device continued to outweigh the risks and the firm’s recall strategy was appropriate and adequate,” Harrison mentioned.
The FDA opinions the recall methods that producers suggest and sometimes gives enter to make sure the general public might be protected, Hils mentioned. The company additionally screens the effectiveness of recollects and, earlier than terminating them, makes positive the technique was carried out, Hils mentioned.
Abbott, the maker of MitraClip, mentioned the gadget has been confirmed secure and efficient “based on more than 20 years of clinical evidence and has profoundly improved the lives of people living with mitral regurgitation,” a situation during which blood flows backward by the guts’s mitral valve. The situation can result in coronary heart failure and loss of life.
“With MitraClip, we’re addressing the needs of people with MR who often have no other options,” firm spokesperson Brent Tippen mentioned.
Talking of the MitraClip recollects, Redberg mentioned, “So hard to imagine these are effective actions in protecting patients.”
In 2021, for Medtronic’s StealthStation S7 cranial software program, the corporate and the FDA despatched a unique message.
StealthStation is an elaborate system of screens and different gear that guides neurosurgeons utilizing devices within the mind — as an illustration, to biopsy or lower out tumors. Drawing from CT scans, MRIs, and different imaging, it’s meant to indicate the situation of the surgical devices.
In reference to a Class I November 2021 recall, the FDA web site mentioned potential inaccuracies in a biopsy depth gauge may end in “life-threatening injury (such as hemorrhage, unintended tissue damage, or permanent neurological injury), which could lead to death.”
The FDA web site defined what Medtronic was doing about it.
“The recalling firm will provide a warning and instructional placard to be applied to impacted systems,” the web site mentioned. “Until a software update is available, ensure you are following the instructions below to prevent the issue from occurring,” it suggested docs.
In an announcement to KFF Well being Information, Medtronic spokesperson Erika Winkels mentioned the security and well-being of sufferers is the corporate’s major concern, and sure points “can be safely and effectively remedied with a correction on site.”
Richard Everson, a neurosurgeon and an assistant professor at UCLA, famous that the 2021 recall allowed docs to proceed utilizing unaffected StealthStation options, a profit for sufferers and amenities relying on them.
“But, I mean, then you could ask, ‘Well, why don’t they just disable the view [of the brain] that’s bugged?’” Everson mentioned. “Why would they give you the option of looking at an inaccurate one?”
“That’s kind of a strange solution,” he mentioned.
The FDA lists the 2021 recall as nonetheless open, explaining “not all products have been corrected or removed.”
That recall was not the final phrase on issues with StealthStation. Since then, the producer has submitted adversarial occasion stories to the FDA describing hassle in instances involving numerous variations of StealthStation.
In a September 2022 case, steerage supplied by a StealthStation gadget was allegedly off the mark, a process was aborted, and, when the affected person awoke, they “had almost no speech for two days,” in accordance with a Medtronic report. Within the report, Medtronic mentioned there was “insufficient information to determine the relationship of the software to the reported issue.”
In a February 2024 case, after mind surgical procedure, an MRI discovered that the operation “missed the tumor” and that different tissue was eliminated as a substitute, in accordance with a report Medtronic submitted to the FDA. Within the report, Medtronic mentioned that when an organization consultant examined the system, it carried out as meant.
In March 2024, Medtronic recalled variations of StealthStation S8 with out eradicating them from hospitals. The corporate mentioned on the time that it could present a software program replace.
“Software updates are available to correct the anomalies identified in the 2021 S7 and 2024 S8 recalls and are actively being deployed,” Medtronic’s Winkels informed KFF Well being Information in a July electronic mail. “While the software updates for the 2021 S7 recall are complete in the US, they remain ongoing in some international regions.”
In June 2023, Abiomed issued an pressing medical gadget correction for its Impella 2.5 intravascular micro axial blood pump, which helps the guts. In sufferers with a sure kind of substitute coronary heart valve, there was a danger of “destruction of the impeller blades,” which may trigger “low flow” and “embolization of the fractured impeller material,” an entry on the FDA web site mentioned.
“Clinicians are cautioned to position the Impella system carefully in patients,” the FDA web site mentioned, amongst different directions.
The up to date directions “provide technical guidance to mitigate the risk of rare complications,” Abiomed spokesperson Ryan Carbain mentioned. There have been no product removals and no stories of adversarial occasions “related to product design or manufacturing,” Carbain mentioned.
One other set of medical gadgets, Cardiosave Hybrid and Rescue Intra-Aortic Balloon Pumps made by Getinge of Sweden, have failed persistently, in accordance with FDA information.
The gadgets — that are positioned within the aorta, a significant artery, to help the guts — had been the topic of eight Class I recollects from December 2022 to July 2023. All had been corrections moderately than removals, a KFF Well being Information evaluation discovered.
In a Might 2024 letter to well being care suppliers, the FDA mentioned that, within the earlier 12 months, it had obtained nearly 3,000 adversarial occasion stories associated to the balloon pumps. It was referring to stories of malfunctions and instances during which the merchandise might need triggered or contributed to a loss of life or damage. Of these, 15 reportedly concerned severe damage or loss of life, the FDA mentioned.
Through the summer time of 2023, the FDA famous that “alternative treatments are limited” and mentioned the gadgets may proceed for use.
However, in Might, the FDA modified its stance. The company suggested well being care amenities to “transition away from these devices and seek alternatives, if possible.”
“These recommendations are based on our continued concerns” that the producer “has not sufficiently addressed the problems and risks with these recalled devices.”
Getinge despatched KFF Well being Information written solutions from Elin Frostehav, the corporate’s president of Acute Care Therapies.
“There is no question that we would have liked to have solved these issues in full much earlier,” she mentioned.
Because of the FDA’s Might motion, the corporate “immediately paused proactive marketing” of the balloon pumps in the USA, and it’s promoting them solely to prospects who don’t have any options, Frostehav mentioned.
“We are working with the agency to finalize remediation and product update solutions,” Frostehav mentioned.
‘Known Possible Complications’
Abbott’s MitraClip system consists of tiny clips implanted within the coronary heart’s mitral valve and the gear used to implant them. The equipment contains a steering mechanism with hand controls and a catheter that’s threaded by a significant vein, usually from an incision within the groin, to position a number of clips within the coronary heart.
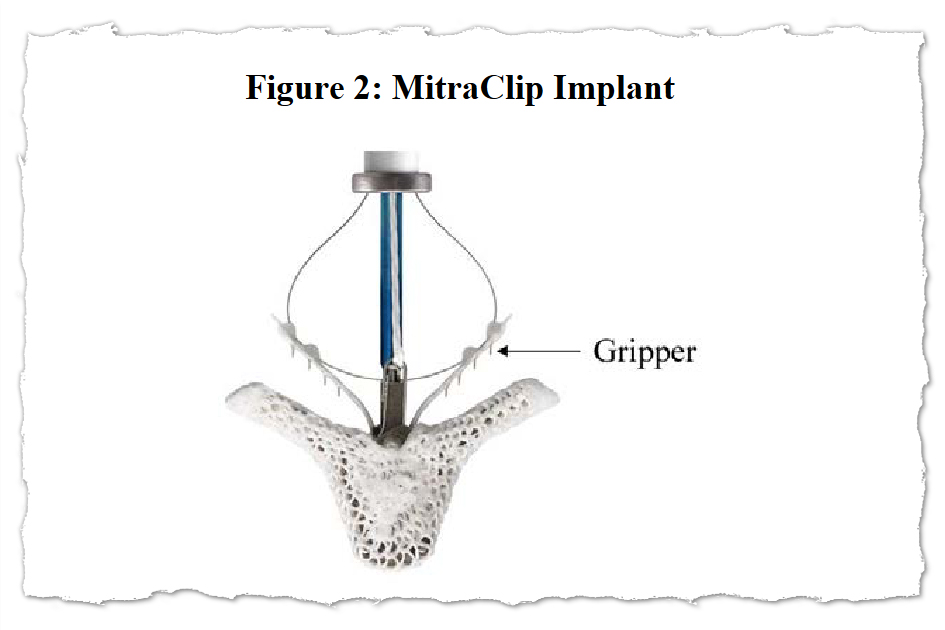
Worldwide, greater than 200,000 individuals have been handled with MitraClip, in accordance with an Abbott web site.
The 2016 MitraClip recall described instances during which “the user was unable to separate the implantable Clip from the delivery system.”
In a information launch on the time, Abbott mentioned it had “received a small number of reports” during which that occurred.
These instances “resulted in surgical interventions to remove the delivery system or replace the mitral valve, and it is expected that any future similar incidents would also require surgery to correct the problem,” the FDA mentioned in a 2016 discover. “There was one patient death in these cases as a result of severe comorbidities following surgery.”
Years later, one thing comparable occurred.
In February 2021, a clip was implanted in an 81-year-old affected person however the physician couldn’t separate the clip from the supply system, in accordance with a report Abbott filed with the FDA. The affected person was transferred to surgical procedure, the place the supply system “had to be cut down in order to detach the clip.”
The affected person then underwent an operation to interchange the mitral valve, and, hours later, the affected person was introduced again to surgical procedure to deal with bleeding, the report mentioned.
The affected person “coded” the subsequent day and died from an aortic bleed, the report mentioned.
Within the report back to the FDA, the producer blamed “case-specific circumstances.”
“Cardiac arrest, hemorrhage and death are listed” within the gadget directions “as known possible complications associated with mitraclip procedures,” the corporate mentioned. “There is no indication of a product issue with respect to manufacture, design or labeling.”
The third MitraClip recall, initiated in September 2022, cited an “increase in clip locking malfunctions.”
Many of the reported malfunctions weren’t related to adversarial outcomes, the FDA mentioned then. Therapy with MitraClip “remains within the anticipated risk levels,” the corporate informed prospects.
As with the 2 earlier recollects, the third suggested docs to comply with the gadget’s directions. However the 2022 recall recognized a contributing issue: the best way the gadget was made.
“Abbott has identified a contributing cause … as a change in the material properties of one of the Clip locking components,” the corporate mentioned in a 2022 letter to prospects.
“Abbott is working on producing new lots with updated manufacturing processing and raw material,” the corporate wrote. In the identical letter, Abbott informed docs that, within the meantime, they may use the gadgets they’d in inventory.
Six days later, a clip opened whereas locked and a affected person died, in accordance with a report the producer submitted to the FDA.
“There is no evidence that death was related to the device but it was likely related to the procedure,” Abbott wrote.
Now, nearly two years later, the 2022 recall stays open, in accordance with the FDA web site, and “not all products have been corrected or removed.”
KFF Well being Information knowledge editor Holly Okay. Hacker contributed to this report.
In the event you’ve had an expertise with a medical gadget and want to inform KFF Well being Information about it, click on right here to contact our reporting staff.




