In 2013, the FDA authorized an implantable machine to deal with leaky coronary heart valves. Amongst its inventors was Mehmet Oz, the previous tv persona and former U.S. Senate candidate broadly often called “Dr. Oz.”
In on-line movies, Oz has referred to as the method that introduced the MitraClip machine to market an instance of American drugs firing “on all cylinders,” and he has in contrast it to “landing a man on the moon.”
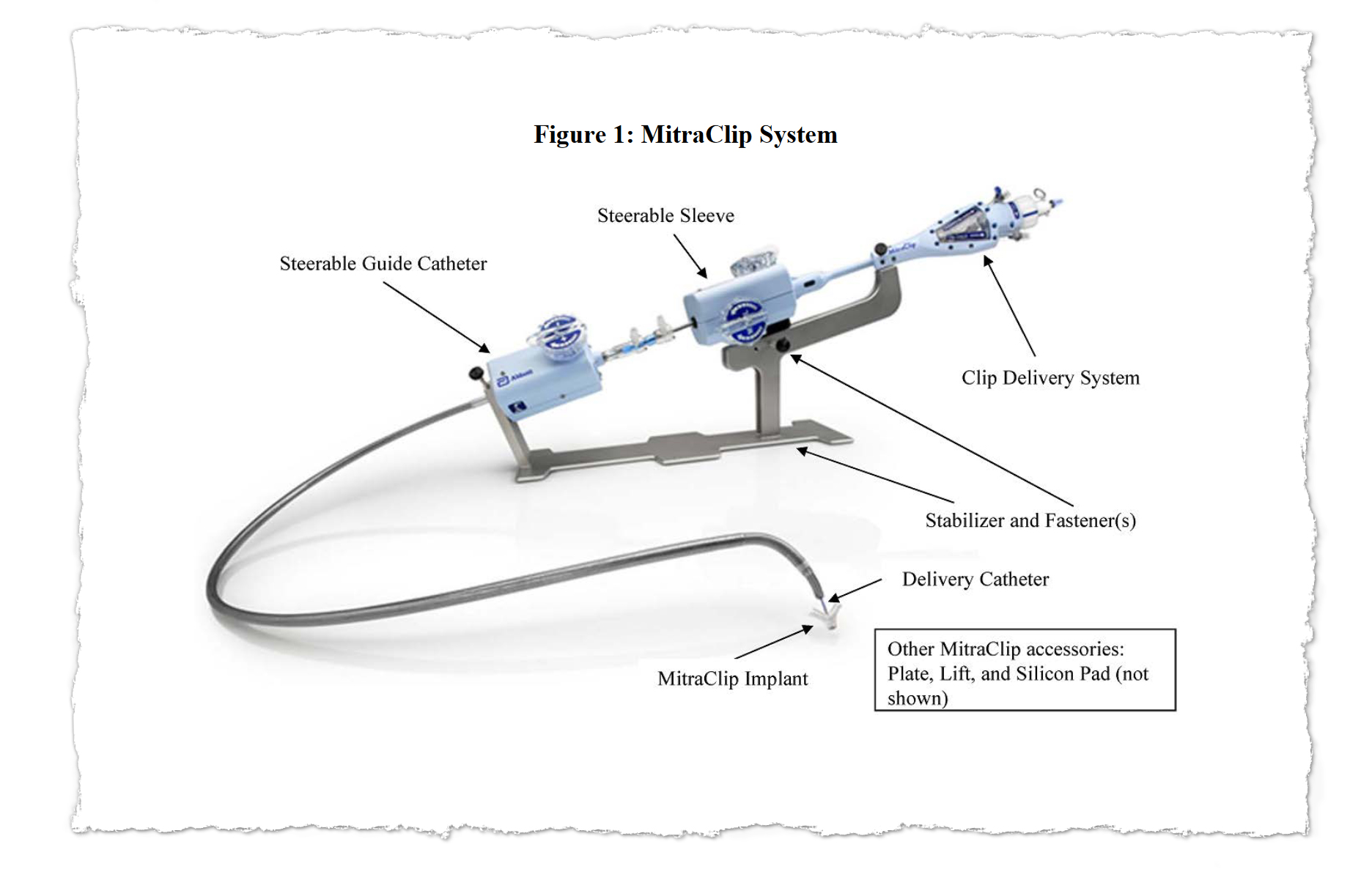
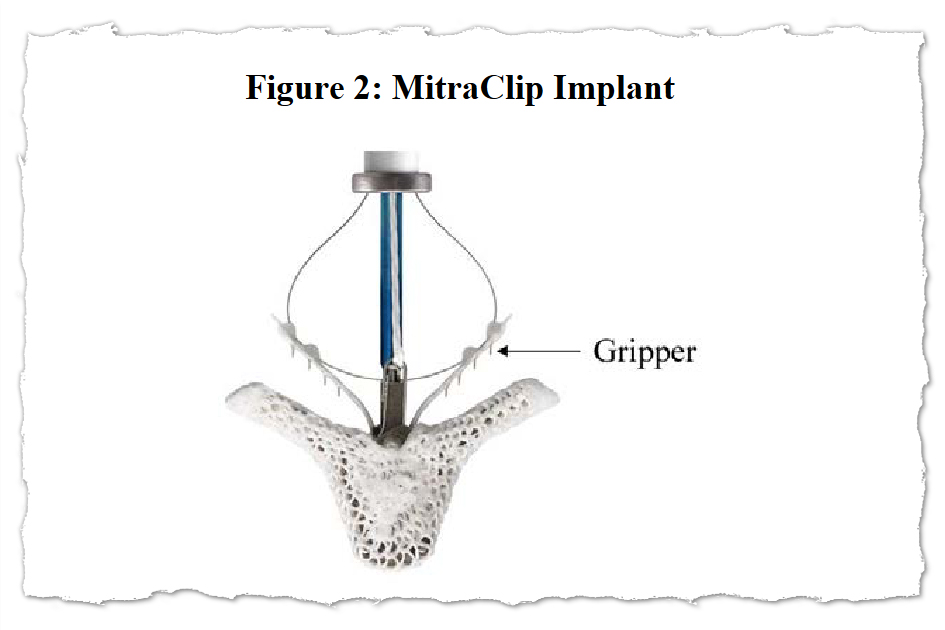
MitraClip was designed to spare sufferers from open-heart surgical procedure by snaking {hardware} into the center via a significant vein. Its producer, Abbott, stated it provided new hope for individuals severely ailing with a situation referred to as mitral regurgitation and too frail to endure surgical procedure.
“It changed the face of cardiac medicine,” Oz stated in a video.
However since MitraClip received FDA approval, variations of the machine have been the topic of 1000’s of studies to the company about malfunctions or affected person accidents, in addition to greater than 1,100 studies of affected person deaths, FDA information present. Merchandise within the MitraClip line have been the topic of three recollects. A former worker has alleged in a federal lawsuit that Abbott promoted the machine via unlawful inducements to docs and hospitals. The case is pending, and Abbott has denied illegally advertising and marketing the machine.
The MitraClip story is, in some ways, a cautionary story in regards to the science, enterprise, and regulation of medical gadgets.
Producer-sponsored analysis on the machine has lengthy been questioned. In 2013, an out of doors adviser to the FDA in contrast a few of the information marshaled in assist of its approval to “poop.”
The FDA expanded its approval of MitraClip to a wider set of sufferers in 2019, primarily based on a medical trial through which Abbott was deeply concerned and regardless of conflicting findings from one other research.
Within the three recollects, the primary of which warned of doubtlessly lethal penalties, neither the producer nor the FDA withdrew stock from the market. The corporate advised docs it was OK for them to proceed utilizing the recalled merchandise.
In response to questions for this text, each Abbott and the FDA described MitraClip as secure and efficient.
“With MitraClip, we’re addressing the needs of people with MR who often have no other options,” Abbott spokesperson Brent Tippen stated. “Patients suffering from mitral regurgitation have severely limited quality of life. MitraClip can significantly improve survival, freedom for hospitalization and quality of life via a minimally invasive, now common procedure.”
An FDA spokesperson, Audra Harrison, stated affected person security “is the FDA’s highest priority and at the forefront of our work in medical device regulation.”
She stated studies to the FDA about malfunctions, accidents, and deaths that the machine could have triggered or contributed to are “consistent” with research outcomes the FDA reviewed for its 2013 and 2019 approvals.
In different phrases: They have been anticipated.
Inspiration in Italy
When an individual has mitral regurgitation, blood flows backward via the mitral valve. Extreme instances can result in coronary heart failure.
With MitraClip, flaps of the valve — often called “leaflets” — are clipped collectively at a number of factors to realize a tighter seal after they shut. The clips are deployed by way of a catheter threaded via a significant vein, usually from an incision within the groin. The process gives a substitute for connecting the affected person to a heart-lung machine and repairing or changing the mitral valve in open-heart surgical procedure.
Oz has stated in on-line movies that he bought the concept after listening to a physician describe a surgical approach for the mitral valve at a convention in Italy. “And on the way home that night, on a plane heading back to Columbia University, where I was on the faculty, I wrote the patent,” he advised KFF Well being Information.
A patent obtained by Columbia in 2001, certainly one of a number of related to MitraClip, lists Oz first among the many inventors.
However a Silicon Valley-based startup, Evalve, would develop the machine. Evalve was later acquired by Abbott for about $400 million.
“I think the engineers and people at Evalve always cringe a little bit when they see Mehmet taking a lot of, you know, basically claiming responsibility for what was a really extraordinary team effort, and he was a small to almost no player in that team,” one of many firm’s founders, heart specialist Fred St. Goar, advised KFF Well being Information.
Oz didn’t reply to a request for touch upon that assertion.
As of 2019, the MitraClip machine value $30,000 per process, in line with an article in a medical journal. Based on the Abbott web site, greater than 200,000 individuals around the globe have been handled with MitraClip.
Oz filed a monetary disclosure throughout his unsuccessful run for the U.S. Senate in 2022 that confirmed him receiving a whole lot of 1000’s of {dollars} in annual MitraClip royalties.
Abbott not too long ago acquired FDA approval for TriClip, a variation of the MitraClip system for the center’s tricuspid valve.
Endorsed ‘With Trepidation’
Earlier than the FDA stated sure to MitraClip in 2013, company staffers pushed again.
Abbott had initially needed the machine authorized for “patients with significant mitral regurgitation,” a comparatively broad time period. After the FDA objected, the corporate narrowed its proposal to sufferers at too-high threat for open-heart surgical procedure.
Even then, in an evaluation, the FDA recognized “fundamental” flaws in Abbott’s information.
One instance: The info in contrast MitraClip sufferers with sufferers who underwent open-heart surgical procedure for valve restore — however the comparability might need been biased by variations within the experience of docs treating the 2 teams, the FDA evaluation stated. Whereas MitraClip was implanted by a extremely choose, skilled group of interventional cardiologists, most of the docs doing the open-heart surgical procedures had carried out solely a “very low volume” of such operations.
FDA “approval is not appropriate at this time as major questions of safety and effectiveness, as well as the overall benefit-risk profile for this device, remain unanswered,” the FDA stated in a evaluation ready for a March 2013 assembly of a committee of out of doors advisers to the company.
Some committee members expressed misgivings. “If your right shoe goes into horse poop and your left shoe goes into dog poop, it’s still poop,” cardiothoracic surgeon Craig Selzman stated, in line with a transcript.
The committee voted 5-4 towards MitraClip on the query of whether or not it proved efficient. However members voted 8-0 that they thought of the machine secure and 5-3 that the advantages of the machine outweighed its dangers.
Selzman voted sure on the final query “with trepidation,” he stated on the time.
In October 2013, the FDA authorized the MitraClip Clip Supply System for a narrower group of sufferers: these with a selected sort of mitral regurgitation who have been thought of a surgical procedure threat.
“The reality is, there is no perfect procedure,” stated Jason Rogers, an interventional heart specialist and College of California-Davis professor who’s an Abbott marketing consultant. The corporate referred KFF Well being Information to Rogers as an authority on MitraClip. He referred to as MitraClip “extremely safe” and stated some sufferers handled with it are “on death’s door to begin with.”
“At least you’re trying to do something for them,” he stated.
Conflicting Research
In 2019, the FDA expanded its approval of MitraClip to a wider set of sufferers.
The company primarily based that call on a medical trial in the US and Canada that Abbott not solely sponsored but in addition helped design and handle. It participated in web site choice and information evaluation, in line with a September 2018 New England Journal of Drugs paper reporting the trial outcomes. Among the authors acquired consulting charges from Abbott, the paper disclosed.

A separate research in France reached a distinct conclusion. It discovered that, for some sufferers who match the expanded profile, the machine didn’t considerably scale back deaths or hospitalizations for coronary heart failure over a yr.
The French research, which appeared within the New England Journal of Drugs in August 2018, was funded by the federal government of France and Abbott. As with the North American research, a few of the researchers disclosed that they had acquired cash from Abbott. Nevertheless, the write-up within the journal stated Abbott performed no position within the design of the French trial, the number of websites, or in information evaluation.
Gregg Stone, one of many leaders of the North American research, stated there have been variations between sufferers enrolled within the two research and the way they have been medicated. As well as, outcomes have been higher within the North American research partly as a result of docs within the U.S. and Canada had extra MitraClip expertise than their counterparts in France, Stone stated.
Stone, a medical trial specialist with a background in interventional cardiology, acknowledged skepticism towards research sponsored by producers.
“There are some individuals who say, ‘Oh, well, you know, these results may have been manipulated,’” he said. “But I can guarantee you that’s not the reality.”
‘Nationwide Scheme’

A former Abbott worker alleges in a lawsuit that after MitraClip received approval, the corporate promoted the machine to docs and hospitals utilizing inducements corresponding to free advertising and marketing assist, the possibility to take part in Abbott medical trials, and funds for taking part in “sham speaker programs.”
The previous worker alleges that she was instructed to inform referring physicians that in the event that they noticed mitral regurgitation of their sufferers to “just send it” for a MitraClip process as a result of “everything can be clipped.” She additionally alleges that, utilizing a script, she was advised to advertise the machine to hospital directors primarily based on monetary benefits corresponding to “growth opportunities through profitable procedures, ancillary tests, and referral streams.”
The inducements have been a part of a “nationwide scheme” of unlawful kickbacks that defrauded authorities medical insurance applications together with Medicare and Medicaid, the lawsuit claims.
The corporate denied doing something unlawful and stated in a courtroom submitting that “to help its groundbreaking therapy reach patients, Abbott needed to educate cardiologists and other healthcare providers.”
These efforts are “not only routine, they are laudable — as physicians cannot use, or refer a patient to another doctor who can use, a device that they do not understand or in some cases even know about,” the corporate stated within the submitting.
Beneath federal legislation, the one who filed the go well with can obtain a share of any cash the federal government recoups from Abbott. The go well with was filed by an organization related to a former worker in Abbott’s Structural Coronary heart Division, Lisa Knott. An lawyer for the corporate declined to remark and stated Knott had no remark.
Studies to the FDA
As docs began utilizing MitraClip, the FDA started receiving studies about malfunctions and instances through which the product might need triggered or contributed to a loss of life or an harm.
Based on some studies, clips indifferent from valve flaps. Flaps turned broken. Procedures have been aborted. Mitral leakage worsened. Medical doctors struggled to manage the machine. Clips turned “entangled in chordae” — cord-like constructions also called heartstrings that join the valve flaps to the center muscle. Sufferers handled with MitraClip underwent corrective operations.

As of March 2024, the FDA had acquired greater than 17,000 studies documenting greater than 22,000 “events” involving mitral valve restore gadgets, FDA information reveals. All however about 200 of these studies point out one iteration of MitraClip or one other, a KFF Well being Information evaluation of FDA information discovered.
Nearly all of the studies got here from Abbott. The FDA requires producers to submit studies after they study of mishaps doubtlessly associated to their gadgets.
The studies should not proof that gadgets triggered issues, and the identical occasion could be reported a number of occasions. Different occasions could go unreported.
Regardless of the studies’ limitations, the FDA supplies an evaluation of them for the general public on its web site.
MitraClip’s dangers weren’t a shock.
Just like the rapid-fire tremendous print in tv adverts for prescribed drugs, the unique product label for the machine listed greater than 60 sorts of potential problems.
Certainly, throughout medical analysis on the machine, about 6% of sufferers implanted with MitraClip died inside 30 days, in line with the label. Nearly 1 in 4 — 23.6% – have been lifeless inside a yr.
The FDA spokesperson, Harrison, pointed to a research initially printed in 2021 in The Annals of Thoracic Surgical procedure, primarily based on a central registry of mitral valve procedures, that discovered decrease charges of loss of life after MitraClip went in the marketplace.
“These data confirmed that the MitraClip device remains safe and effective in the real-world setting,” Harrison stated.
However the research’s authors, a number of of whom disclosed monetary or different connections to Abbott, stated information was lacking for greater than 1 / 4 of sufferers one yr after the process.
A significant measure of success can be the proportion of MitraClip sufferers who’re alive “with an acceptable quality of life” a yr after present process the process, the research stated. As a result of such data was out there for fewer than half of the residing sufferers, “we have omitted those outcomes from this report,” the authors wrote.
In the event you’ve had an expertise with MitraClip or one other medical machine and wish to inform KFF Well being Information about it, click on right here to share your story with us.
KFF Well being Information viewers engagement producer Tarena Lofton contributed to this report.




